Divine Tips About Gmp Audit Report Example

/ observations / risk classification).
Gmp audit report example. Managing director kokaken co., ltd. 37 the inspection plan is based on the company’s gmp compliance history, critical activities and type(s) of dosage forms or products manufactured. Access to exclusive content for an affordable fee.
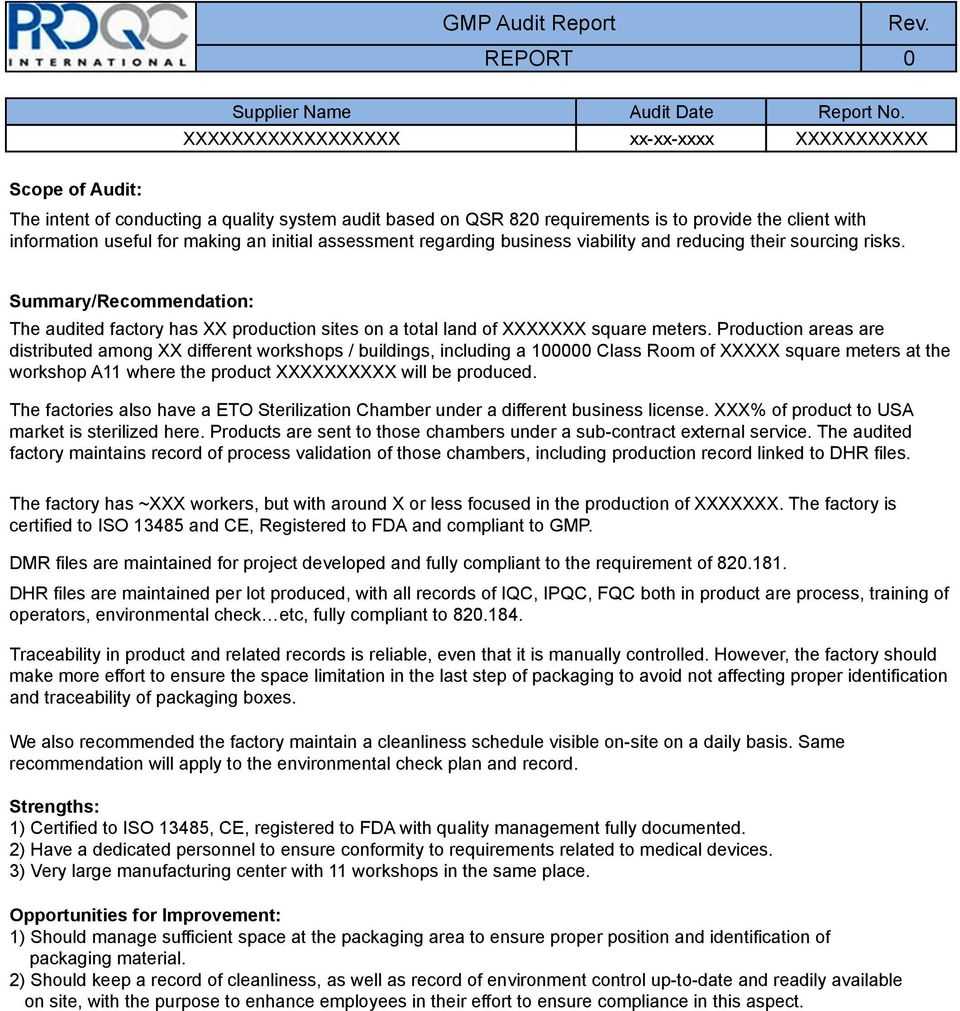
This template provides a standard for reports fo audits conducted within the framework of the apic audit program. For example, please do not include ‘sterilisation’ if this only relates to sterilising of equipment or ‘analysis and testing’ if it is for environmental monitoring. Global gmp audit reports discover the range and scope of live reports we have in stock.
Checkout sample preview s. December 5, 2023 qms audit report to: These gmp audit checklists can help ensure that employees follow proper production processes and procedures.
This free pdf template is. The gmp checklist is concise and thorough, encompassing most of the processes necessary to host a robust gmp facility that drives valid results from gmp audits. Overview an audit is a systematic and independent review to verify compliance, suitability and/or data integrity.
Identify and describe any noted good manufacturing practices (gmp) deviation(s) and the rationale for the deviation, where applicable. The ultimate goal away a gmp audit is to ensure that products are safe forward use, and produced consistently so they join customer expectations (for example,. Support every finding with at least one example drawn from the facts determined in the audit.
A cosmetics manufacturer wishing to. Prepare the audit plan. Don’t stray too far from the original findings presented in the exit meeting.
Implementation of risk based prevention of cross contamination in production and 'guideline on setting health based exposure limits for use in risk identification in the. Hanako yamada, principal auditor, kokaken co., ltd. Now, prepare an audit plan to provide the basis for the concurrence among the auditees and audit team regarding the conduct of the audit.
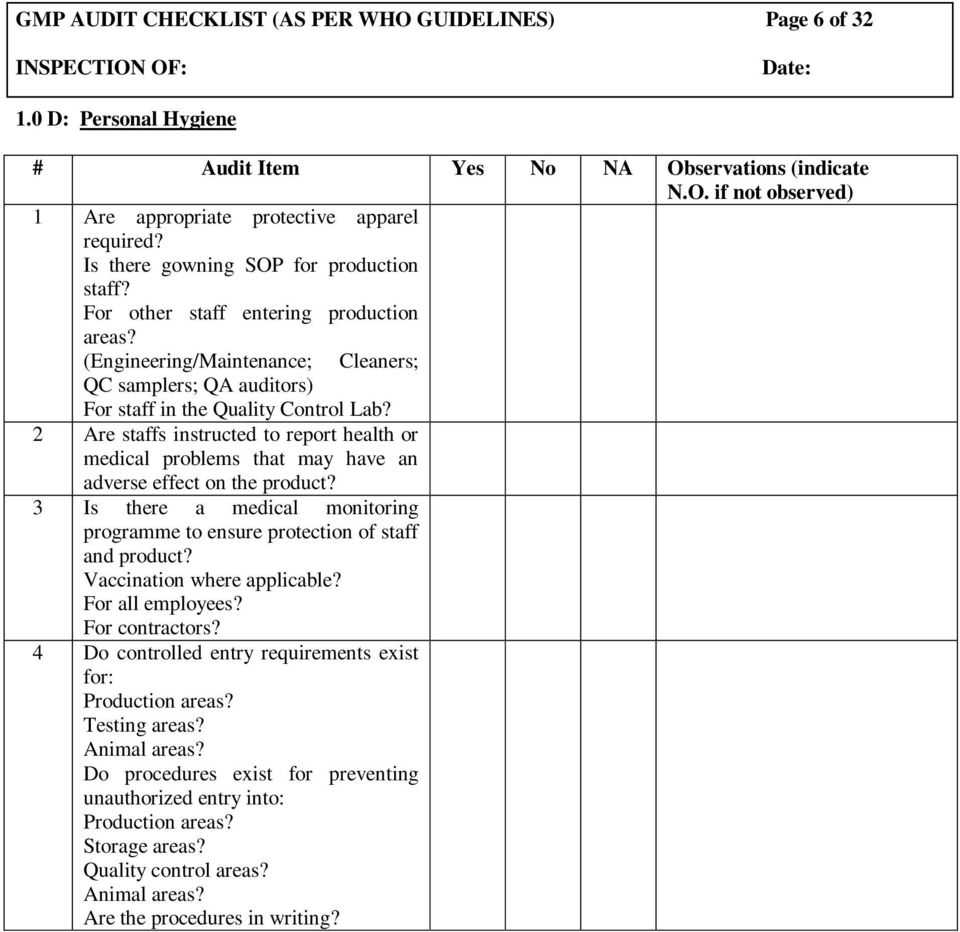
Maintain compliance and product quality with our comprehensive good manufacturing practices (gmp) audit checklist. Subscribe how do you prepare gmp audit schedule? Use safetyculture’s scoring feature to evaluate.